Why ROTOP?
We support your radiopharmaeutical journey with:
What we do
We provide end-to-end CDMO services for radiopharmaceuticals — from early-stage development to commercial supply — all under one roof.
Our Services
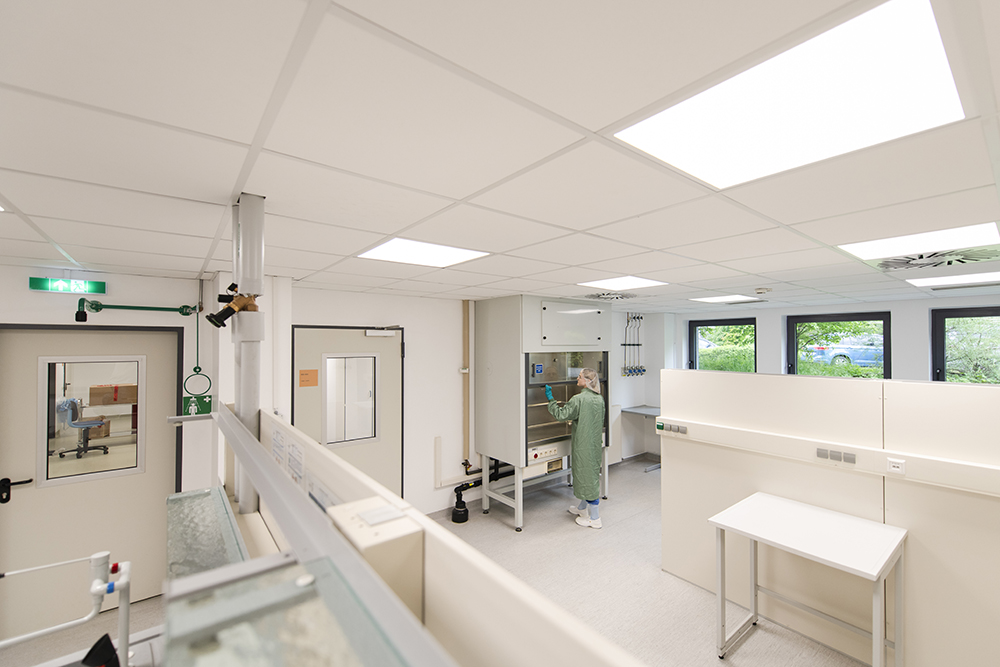
Our facilities and capabilities
Our facilities are purpose-built for radiopharmaceutical development and manufacturing, offering:
- ■ Hot cells: Safe handling of alpha, beta and gamma emitters
- ■ Cleanrooms: Modular GMP suites for clinical and commercial batches
- ■ QC Labs: Radiochemical, microbiological and method validation
- ■ Precursor labs: Custom synthesis and scale-up optimization
- ■ Non-GMP areas: Early feasibility and process development
19,000
sqft lab & clean room space
5,000
vials per batch possible
10+
CDMO projects successfully completed
CDMO Services in focus
Hot Cell Manufacturing
GMP Manufacturing
Precursor Laboratories
-
- ■ Clinical & commercial batch manufacturing
- ■ Multiple isotope support (e.g. Ac-225, Cu-64, Ga-68, I-123, Lu-177, Pb-212)
- ■ I-123 Iodide solution supply for radioactive compound development
- ■ Just-in-time global logistics
- ■ Early market access of clinical products under special national programs
-
-
- ■ Formulation & lyo cycle development
- ■ Radiolabeling and QC method validation
-
- ■ Transfer to GMP suite for clinical manufacturing
- ■ In-process control definition (e.g. CCIT)
- ■
- ■ 100% visual inspection and GDP stability testing
-
- ■ Synthesis of peptides and small molecules
- ■ GMP Manufacturing and aliquotation services
- ■ Process optimization using Quality by Design (QbD) principles
- ■ Stability studies and scale-up support
Hot Cell Manufacturing
-
- ■ Clinical & commercial batch manufacturing
- ■ Multiple isotope support (e.g. Ac-225, Cu-64, Ga-68, I-123, Lu-177, Pb-212)
- ■ I-123 Iodide solution supply for radioactive compound development
- ■ Just-in-time global logistics
- ■ Early market access of clinical products under special national programs
GMP Manufacturing
-
-
- ■ Formulation & lyo cycle development
- ■ Radiolabeling and QC method validation
-
- ■ Transfer to GMP suite for clinical manufacturing
- ■ In-process control definition (e.g. CCIT)
- ■
- ■ 100% visual inspection and GDP stability testing
-
Precursor Laboratories
- ■ Synthesis of peptides and small molecules
- ■ GMP Manufacturing and aliquotation services
- ■ Process optimization using Quality by Design (QbD) principles
- ■ Stability studies and scale-up support
Strategic Location
Headquartered in Dresden, Germany, with reliable worldwide logistics via Berlin and Prague International Airports and Leipzig DHL Hub (3rd largest hub in Europe) for temperature-sensitive, just-in-time shipments.
Our Team
At ROTOP, people drive innovation. Our multidisciplinary CDMO team blends radiopharmaceutical expertise, regulatory insight and global development experience.
Meet the leadership:
- Dr. Thomas Gottlieb – Chief Business Officer
- Andrew Varghese – President, ROTOP US & CDMO Services
- Dr. Tanmaya Joshi – Executive VP (CDMO)
Together they bring decades of experience in theranostics, CDMO strategy and global pharma leadership – ensuring that your project is in expert hands.
We're more than a manufacturer – we're your development partner. From concept to commercialization, we're here to accelerate your success.
We believe in long-term, transparent collaboration built on trust and shared goals. With every CDMO project, we aim to drive sustainable innovation that advances patient care and creates lasting value — for you, and for those who depend on your therapies.
Let’s collaborate